Uranium, classified as a chemical element, is identified by the symbol U and has an atomic mass of 238. In nature, it is found in the form of three isotopes: U234, U235, U238. It is the natural element with the highest atomic number. It is surpassed only by artificial radioactive elements (transuranic) such as neptunium and plutonium. It is a radioactive element and belongs to the group of actinides.
Uranium is a very common element on Earth, incorporated into the planet during its formation, being considered more abundant than mercury, antimony, silver and cadmium and as abundant as molybdenum and arsenic.
It is believed to be the product of the decay of elements of even higher atomic numbers, which existed at some time in the Universe. It can be recognized as a silvery radioactive metal, malleable, ductile and less hard than steel, which, when exposed to air, forms a layer of oxide on its surface.
Uranium can be found in several types of deposits. The most common and important form of oreis uraninite, composed of a mixture of UO2 with U3O8. Other minerals that contain uranium are autunite, carnotite, branerite, torbernite, among others.
Its main commercial application is the generation of electrical energy, as a fuel for nuclear power reactors. To do so, it goes through a series of stages and processes, including mining, processing and production of the fuel element, composed of uranium dioxide pellets.
Geological context and forms of occurrence
Uranium ore is known worldwide and explored in rocks of Archean to recent age and in the most distinct geological environments , from deposits related to albitites in Proterozoic rocks, to sands in recent sedimentary basins, superficial placer-type deposits and concentration by weathering processes.
The recently updated IAEA (International Atomic Energy Agency) UDEPO (World Distribution of Uranium Deposits) database includes information on 1,807 known uranium deposits worldwide , 20 of which are in Brazil. The deposits are classified into 15 typologies and 37 subtypologies.
Despite Brazil's large territorial extension and the diversity of geological environments, deposits are known in only 7 of the typologies classified by the IAEA, half of which are related to intrusive rocks or metasomatism.
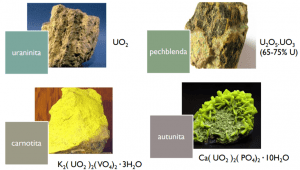
Uranium occurs in several minerals such as uraninite (a complex uranate of uranyl and lead, which may contain lanthanum, thorium, and yttrium. Also called pitchblende), carnotite (potassium and sodium uranovanate), autunite (hydrated calcium uranium phosphate), torbernite (hydrated copper uranium phosphate), and zeunerite (hydrated uranium copper arsenate).



It can also be found in rocks with phosphates , in lignite (fossil coal, intermediate stage between peat and bituminous coal) and in sands with monazite (cerium, lanthanum, praseodymium, neodymium phosphate, with thorium oxide).
Uranium is a silvery metal and its main form of occurrence is uraninite (UO2). Primary or secondary varieties of uranium minerals occur in various colors such as yellow (uranophane, carnotite, autunite) and green (tobernite).
History and Discovery
German pharmacist Martin Klaproth became the first scientist to discover uranium , naming the chemical element after the planet Uranus (the second-to-last planet in the Solar System counting from the Sun). Despite this, its use, at least in oxide form, has been known for a long time, as a yellow glass, dated 79 AD, was found in Naples , Italy containing about 1% uranium oxide.




Later, the element was isolated from the mineral by the Frenchman Eugene-Melchior Peligot in 1841, through the reduction of anhydrous chloride with potassium.
In 1896, Frenchman A. Henri Becquerel discovered that uranium had radioactive properties during an attempt to demonstrate the relationship between X-rays and the luminescence of uranium salts by placing a quantity of uranium salt on a photographic plate wrapped in black paper and exposed to sunlight. After the plate was developed, it was shown that the rays emitted by the salt passed through the black paper .
Later, when repeating the experiment, Becquerel, unable to find good sunlight conditions, stored the material in a drawer. When Becquerel took the material again to resume his research, he observed an intense image on the plate . He repeated the experiment in total darkness, obtaining the same result, proving that the uranium salts emitted rays that affected the photographic plate without it being exposed to sunlight.
In this way Becquerel discovered the radioactivity of uranium . The discovery of nuclear fission by the Germans Otto Hahn and Fritz Strassman in 1939 made uranium an element of great importance.
Uranium as a mineral and energy resource
Uranium ore serves several industrial sectors by supplying raw materials for the steel, automobile, fiber optic and special ceramics industries. Until World War II, uranium ores were only a commercial source of radium (Ra).
Uranium salts had limited applications (photography, ceramics). With the development of the nuclear industry, uranium began to be used in weapons and nuclear reactors . Today, although it is also used in medicine and agriculture, its main commercial application is in the generation of electrical energy , as fuel for nuclear power reactors.




Thus, the global demand for uranium is formed by several countries that use nuclear energy in their energy matrix.
Uranium mineral research
Law 4,118 of 1962 was created to regulate the National Nuclear Energy Policy and create the National Nuclear Energy Commission (CNEN). This law became famous as the “Monopoly Law” , and the reason for this can be seen in its first article:
Art. 1º The following constitute a Union monopoly:
I – Research and mining of nuclear mineral deposits located in the national territory;
II – Trade in nuclear minerals and their concentrates; nuclear elements and their compounds; fissile and fertile materials, artificial and substantial radioisotopes and radioactive substances of the three natural series; nuclear by-products;
III – The production of nuclear materials and their industrialization.
Because this law existed prior to the enactment of the current Constitution , it also contains an exclusive article on the subject:
Art. 177. The following constitute a monopoly of the Union:
V – research, mining, enrichment, reprocessing, industrialization and trade of nuclear ores and minerals and their derivatives, with the exception of radioisotopes whose production, commercialization and use may be authorized under a permit regime, in accordance with items b and c of item XXIII of the caput of art. 21 of this Federal Constitution.
Thus, uranium mineral research and all its stages, in the Brazilian context, are the exclusive responsibility of the Union , in the form of its state-owned nuclear industries.
The monopoly situation in Brazil was not exclusive to uranium and in past decades other sectors faced the same discussion , the most notable being the telecommunications and oil sectors, with oil being in a similar situation to uranium.
The premises of monopoly are enshrined in the Federal Constitution:
Art. 21. The Union is responsible for:
XXIII – explore nuclear services and facilities of any nature and exercise a state monopoly over research, mining, …, subject to the following principles and conditions:
a) all nuclear activity in national territory will only be permitted for peaceful purposes and with the approval of the National Congress;
b) under a concession or permission regime, the use of radioisotopes for research and medicinal, agricultural, industrial and similar uses is authorized;
c) civil liability for nuclear damage is independent of the existence of fault;
Paragraphs b and c are interesting points for examining the legislation and its practical effects.
Briefly and as an example, the legal effect of paragraph c is to make the Union responsible for practically any nuclear accident , whether or not it is imbued with intent by the perpetrator, and this has already had repercussions in serious problems in the Brazilian past, such as the well-known accident with Cesium-137 in Goiás and the suspicions of contamination by radioactivity in the surroundings of state-owned nuclear companies.
Subparagraph b is of greater interest to our sector. After a long battle, the industrial sector, including the agricultural, pharmaceutical and mining industries, managed to break the monopoly on the use of radioisotopes in October 2006, through Constitutional Amendment number 49 of 2006.
This flexibility is extremely important for the use of CT scanners, X-ray machines and similar devices in medical applications. In the food sector, radiation equipment is used to increase the shelf life of food by altering certain organic chains, making them less susceptible to degradation.
In our sector, mining companies use spectrometers with different functionalities such as X-ray fluorescence, atomic absorption and X-ray diffractometers, which use tubes of Thorium, Radium or other radioactive elements and are vital for the chemical, mineralogical and structural analysis of mineral resources.
Technological evolution in the sector now allows chemical analyses to be carried out by sensors installed on conveyor belts, calibrating mineral processing models or even carrying out pre-concentration, as in the case of ore-sorting.
The first national project and the only uranium mine in Latin America
In Brazil, the most important source of nuclear resources is the Lagoa Real Uranus District , which is located in a region marked by past orogenic events in south-central Bahia, approximately 20 km from the city of Caetité.
In addition to this mine, in the past, between 1981 and 1995, the Poços de Caldas mine produced around 1,200 t of uranium concentrate. This mine had an average grade of 800 PPM (parts per million), a much lower quality than that observed in Caetité (with the potential to reach 3,000 PPM).




After a campaign, between 1976 and 1977, of generic aerogeophysical surveys by the Brazilian Geological Service, CPRM – Mineral Resources Research Company – 19 targets with potential for uranium were identified , considered as mineral occurrences.
The studies gained momentum with the creation of NUCLEBRÁS in December 1974 , the advent of the “first oil crisis” in 1973, which boosted the search for new energy sources, and an aerogamma spectrometry study confirming 33 uranium occurrences. The allocation of specific financial resources for the search for uranium increased, coming from the Brazilian Nuclear Program.
The focus included prospecting, research, development of methods and techniques for working and mining uranium deposits in the country, resulting in the discovery of the Itatiaia deposits in Santa Quitéria in Ceará – a deposit with potential for the production of limestone and high-grade phosphate together with uranium – and Lagoa Real in Caetité in Bahia. Only Lagoa Real was converted into a mine and remains in operation.
